Water is often referred to as the essence of life, a universal solvent that plays an integral role in countless chemical processes. Its simple yet profound molecular structure—H2O—is not just a basic building block for living organisms; it serves as a dynamic player in the world of chemistry. But what about more complex compounds like hcooch ch2 h2o? Understanding this compound and its relationship with water can open up fascinating insights into biological systems and chemical reactions. Dive into the intricate dance between water and chemistry, where every drop tells a story of transformation, interaction, and essentiality.
What is hcooch ch2 h2o?
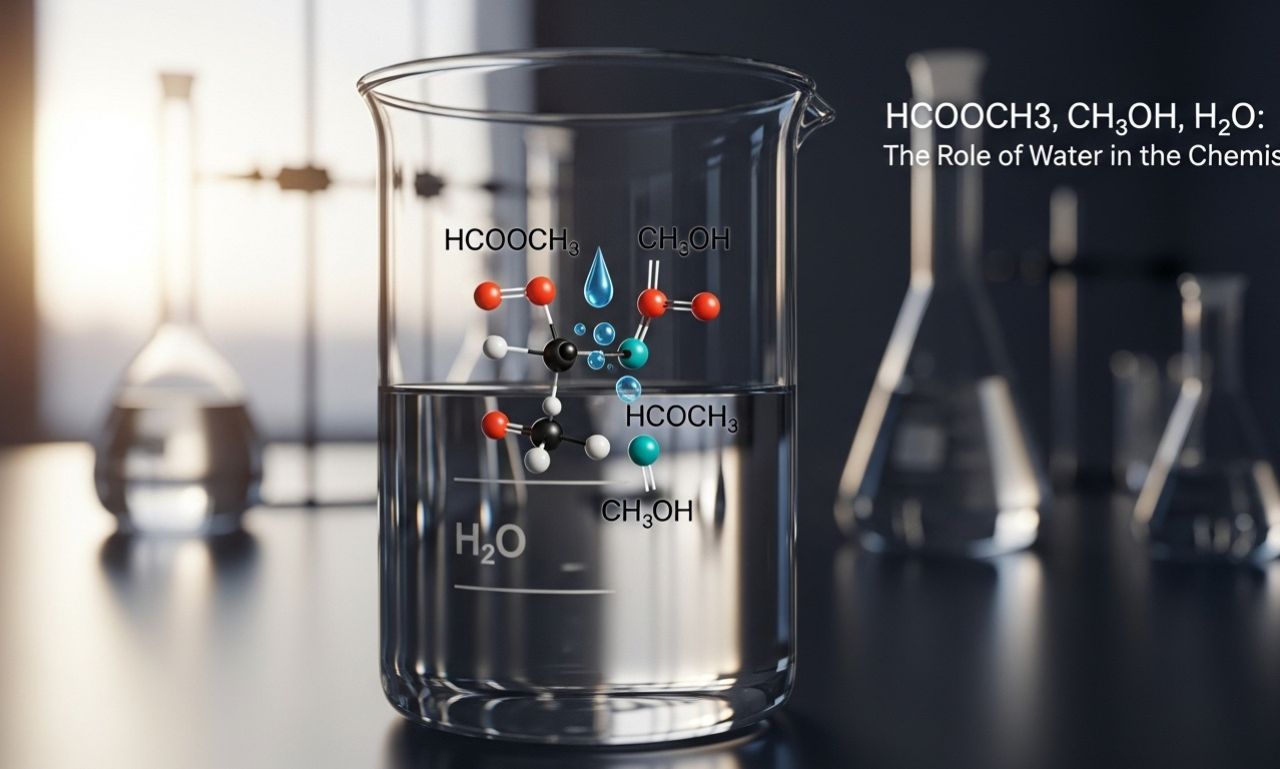
HCOOCH₂H₂O represents a specific chemical structure that highlights the interplay between organic compounds and water. This formula consists of an ester group, which is integral to many biological processes.
The “HCOO” part indicates a formate ion, often associated with various biochemical reactions. The “CH₂” suggests a methylene group that plays a key role in forming connections within larger molecules.
Water (H₂O) is crucial here as it interacts dynamically with these components. Its polar nature allows for unique bonding opportunities and influences molecular behavior significantly.
In essence, HCOOCH₂H₂O exemplifies how organic chemistry intertwines with water’s properties to create functional systems in biology. Understanding this relationship provides insights into metabolic pathways and ecological interactions.
The Importance of Water in Biological Systems
Water is often referred to as the essence of life, playing a critical role in biological systems. It acts as a medium where countless biochemical reactions occur, facilitating everything from metabolism to cellular respiration.
In living organisms, water is vital for maintaining homeostasis. It regulates temperature and maintains hydration levels within cells. This balance ensures that enzymatic reactions can proceed efficiently.
Moreover, water’s unique properties enable it to dissolve various substances, making nutrients accessible. Cells rely on aqueous environments for nutrient absorption and waste elimination.
The transport system within organisms also heavily depends on water. Blood plasma—a major component—is primarily composed of this essential liquid, allowing oxygen and nutrients to reach every cell in the body swiftly.
Plants depend on water for photosynthesis too; it’s not just humans who thrive because of it! Water sustains ecosystems by supporting plant growth and nurturing biodiversity across habitats.
Role of Water as a Solvent
Water is often called the universal solvent, and for good reason. Its unique molecular structure allows it to dissolve a wide range of substances.
When ionic or polar molecules come into contact with water, they interact in fascinating ways. The positive hydrogen atoms attract negatively charged ions, while the oxygen atom draws in positively charged ones.
This capability makes water essential for various biological processes. Nutrients travel through cells and tissues dissolved in water, facilitating vital functions like metabolism and waste removal.
Additionally, its ability to form hydrogen bonds contributes to its effectiveness as a solvent. This creates an environment where reactions can occur more readily.
Water’s role extends beyond mere dissolution; it shapes environments and influences chemical behavior across ecosystems. In countless scenarios—from human physiology to environmental science—water stands out as an irreplaceable player in chemistry’s grand narrative.
Hydrophobic and Hydrophilic Interactions
Hydrophobic and hydrophilic interactions are fundamental concepts in chemistry, particularly when discussing the behavior of molecules in water. Hydrophilic substances love water. They readily dissolve or interact with it due to their polar nature.
Think of salt or sugar; they easily mix with water, showcasing strong attractions between their molecules and water’s hydrogen bonds. This affinity plays a crucial role in various biological processes.
On the other hand, hydrophobic substances repel water. Oils and fats exemplify this characteristic. Their non-polar structures avoid interaction with polar molecules like water, leading to phenomena such as oil droplets forming on the surface.
These opposing behaviors dictate how substances interact within biological systems. They influence cell membrane composition, protein folding, and even enzyme activity. Understanding these principles enriches our grasp of molecular dynamics in watery environments like cells.
Effects of pH on Water’s Chemical Properties
pH plays a crucial role in determining water’s chemical properties. It measures the acidity or alkalinity of a solution, impacting how molecules interact.
When pH levels shift, the balance between hydrogen ions and hydroxide ions changes. This alteration can affect solubility and reactivity of various compounds dissolved in water.
For example, at low pH (acidic conditions), certain minerals become more soluble. Conversely, high pH (alkaline conditions) can precipitate minerals out of solutions.
Biological processes are also influenced by these shifts. Enzymatic reactions often require specific pH ranges for optimal activity.
Thus, understanding how pH affects water is essential for fields like chemistry and biology. The implications reach far beyond simple measurements—affecting ecosystems and human health alike.
Water’s Role in Chemical Reactions
Water is often termed the universal solvent, and for good reason. It plays a pivotal role in countless chemical reactions. This unique characteristic stems from its polar nature, allowing it to interact with various molecules.
In many biochemical processes, water facilitates the transport of ions and other reactants. Its presence can lower activation energy, making reactions occur more readily. For instance, in hydrolysis reactions, water breaks down complex molecules into simpler ones.
Moreover, water contributes to maintaining temperature stability during exothermic or endothermic reactions. It absorbs heat without significant changes in its own temperature.
The dynamics of solvation are also essential in enzyme activity where substrates must dissolve efficiently to bind with active sites effectively. Water’s versatile properties make it an irreplaceable player in chemistry across biological systems and industrial applications alike.
The Essentiality of Water in Chemistry
Water is often referred to as the universal solvent, hcooch ch2 h2o a title that speaks volumes about its significance in chemistry. This simple molecule consists of two hydrogen atoms bonded to one oxygen atom, yet its properties are anything but basic.
In chemical reactions, water facilitates the movement and interaction of molecules. It not only dissolves various substances but also helps maintain optimal conditions for these reactions to occur.
The unique ability of water to stabilize temperature makes it invaluable in processes where heat management is crucial. Its high specific heat capacity allows environments—like living cells—to resist drastic temperature changes.
Moreover, without water, many biochemical pathways would halt entirely. The presence or absence of this vital compound can alter molecular behavior dramatically, influencing everything from metabolic rates to reaction dynamics. Water truly embodies life’s essence at the molecular level within chemistry.
Conclusion
Water is truly remarkable, serving as a vital component in both chemistry and biology. Understanding its structure allows us to appreciate its unique properties. The chemical formula hcooch ch2 h2o highlights the connection between water and organic compounds, illustrating how it interacts at a molecular level.
The importance of water stretches far beyond just being essential for life; it acts as a universal solvent, facilitating countless biochemical reactions. Its ability to engage in hydrophilic and hydrophobic interactions underlines its role in maintaining cellular integrity.
Moreover, pH levels can significantly influence water’s chemical behavior, impacting everything from enzyme activity to metabolic processes. Water doesn’t merely support life; it drives the intricate web of chemical reactions that sustain biological systems.
Whether considering its solvation capabilities or its participation in critical reactions, the essence of water permeates every aspect of chemistry. As science continues to unveil more about this incredible molecule, our appreciation for its role will only deepen further. Water remains indispensable across diverse fields—its versatility is unmatched and continues to inspire research and innovation.