Have you ever wondered about the intricate behavior of compounds in aqueous solutions? If so, hcooch ch2 h2o is a fascinating subject to explore. This compound, often overlooked, plays a significant role in various chemical processes and industries. Understanding its properties can unlock new insights into its applications and effects on our environment. So let’s dive deeper into what HCOOCH2H2O really is and discover why it might matter more than you think!
What is hcooch ch2 h2o?
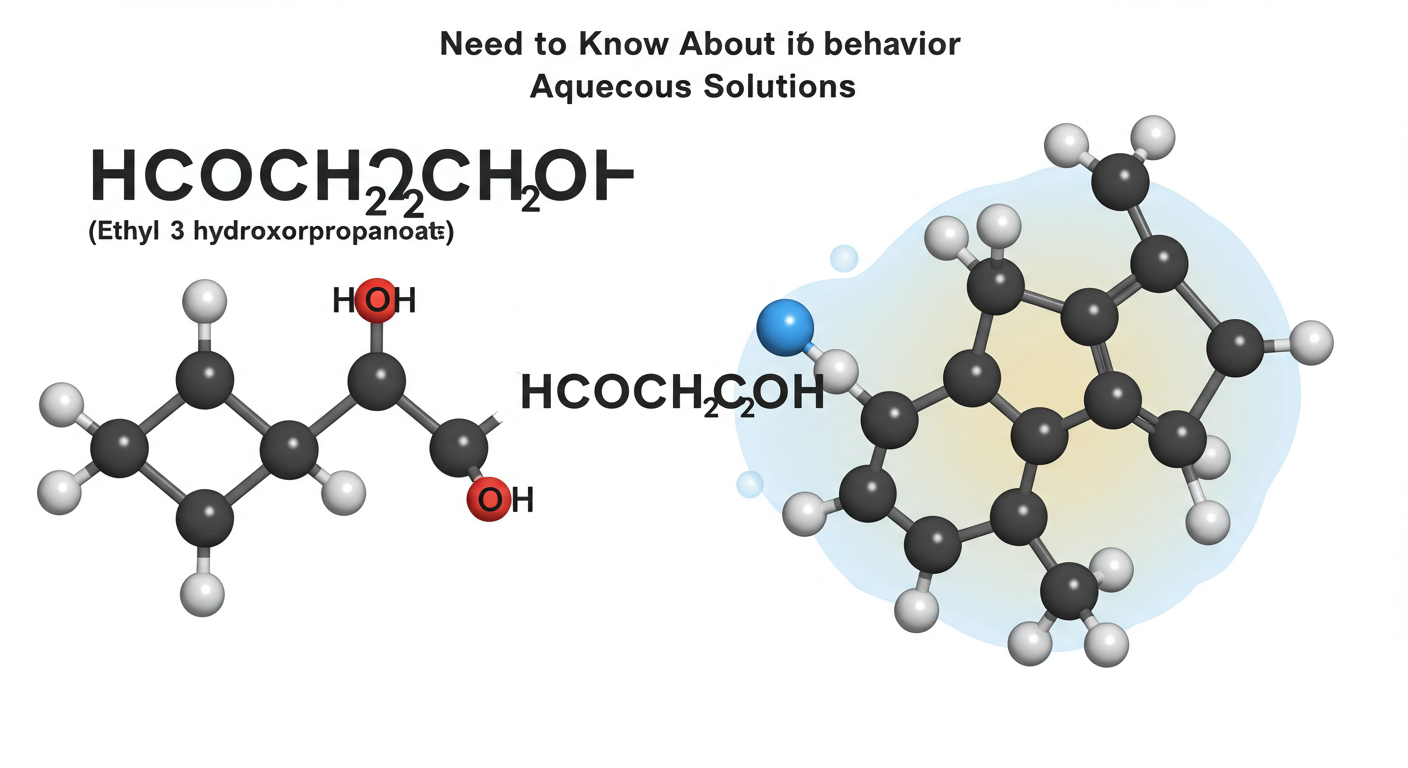
hcooch ch2 h2o, commonly known as formic acid ethylene glycol ester, is an intriguing compound in the realm of chemistry. It features a unique structure that combines both formate and glycol functionalities. This combination imparts specific characteristics that make it useful in various applications.
The formula reflects its composition: one carbonyl group from the formate moiety and two hydroxymethyl groups from ethylene glycol. This gives HCOOCH2H2O distinct properties compared to other esters.
Understanding this compound requires looking at its behavior in different environments, particularly when dissolved in water. Its interactions can lead to interesting reactions, making it a subject of study for chemists interested in organic synthesis and material science.
Due to its dual nature, HCOOCH2H2O finds utility across multiple sectors such as pharmaceuticals and agriculture. Each application highlights how versatile this chemical can be when harnessed effectively.
Chemical Properties of HCOOCH2H20
HCOOCH2H2O, known as formic acid ethylene glycol monomethyl ether, exhibits intriguing chemical properties. It is a colorless liquid with a distinct odor, often used in various industrial applications.
This compound has a molecular weight of approximately 76 grams per mole. Its structure comprises ester functional groups that contribute to its reactivity. HCOOCH2H2O can engage in hydrolysis reactions when exposed to water, leading to the formation of formic acid and other byproducts.
The presence of hydroxyl groups enhances its solubility in polar solvents. This makes it versatile for use in different formulations. Furthermore, HCOOCH2H2O demonstrates moderate volatility at room temperature.
Its interactions with acids and bases are noteworthy too. The behavior under varying pH levels may affect its stability and usability across diverse environments. Such characteristics make it an essential component across multiple sectors.
Solubility of HCOOCH2H20 in Water
HCOOCH2H2O, or formate diethyl glycol, exhibits interesting solubility characteristics in water. Its molecular structure allows it to interact favorably with water molecules, resulting in good solubility.
When introduced to an aqueous environment, HCOOCH2H2O readily dissolves. This behavior is largely attributed to hydrogen bonding between the hydroxyl groups and water.
The presence of both hydrophilic (water-attracting) and hydrophobic (water-repelling) components plays a crucial role in its interaction with water. Consequently, this compound finds applications across various industries due to its effective dissolution properties.
Understanding the solubility of HCOOCH2H20 can significantly impact processes in chemical reactions and formulations where aqueous solutions are involved. It’s essential for industries that rely on consistent mixing and blending for optimal results.
Impact on pH Levels
The impact of HCOOCH2H2O on pH levels is quite fascinating. When introduced into an aqueous solution, it can exhibit weak acidic properties. This behavior is primarily due to its ability to donate protons in specific conditions.
As the concentration increases, there might be noticeable fluctuations in pH. These shifts can affect various chemical reactions and biological processes occurring in the solution.
Moreover, HCOOCH2H2O can interact with other substances present in the water. Such interactions may lead to further changes in acidity or alkalinity, influencing everything from nutrient availability to microbial activity.
Understanding these dynamics is crucial for industries that rely on precise pH control. Whether it’s pharmaceuticals or food production, maintaining optimal conditions ensures product integrity and safety.
Uses of HCOOCH2H20 in Industries
HCOOCH2H2O, also known as formic acid methyl ester hydrate, plays a multifaceted role across various industries. Its unique chemical properties make it valuable in agricultural applications. Farmers utilize it as a pesticide and herbicide due to its efficacy against pests.
In the pharmaceutical sector, HCOOCH2H2O serves as an intermediate in synthesizing active ingredients for medications. Its ability to act as a solvent enhances the solubility of compounds, making drug formulation more efficient.
Moreover, this compound finds utility in textile manufacturing. It helps improve dye uptake and fixation on fabrics, resulting in vibrant colors that are long-lasting.
The food industry also benefits from HCOOCH2H2O’s preservative qualities. It’s used to inhibit microbial growth in various products while maintaining flavor integrity.
These diverse applications underline its importance and adaptability within industrial processes.
Safety Precautions when Handling HCOOCH2H20
When working with HCOOCH2H2O, safety should always be a top priority. Proper personal protective equipment (PPE) is essential. Wear gloves, goggles, and lab coats to minimize exposure.
Ensure that your workspace is well-ventilated. This reduces inhalation risks associated with any fumes released during handling. A fume hood is ideal for such tasks.
Be familiar with the Material Safety Data Sheet (MSDS) for HCOOCH2H2O before starting any work. Understanding its properties will help you respond effectively in case of spills or accidental contact.
Maintain proper storage conditions by keeping the compound sealed and stored away from incompatible materials. Label all containers clearly to avoid confusion later on.
In case of skin or eye contact, rinse immediately with plenty of water and seek medical attention if necessary. Having an emergency plan in place can make a significant difference in ensuring safety during handling activities as well.
Final Thought
The behavior of HCOOCH2H2O in aqueous solutions is a fascinating subject. Its unique properties make it an intriguing compound for study and application.
Understanding its solubility and interactions with water opens doors to various industrial uses.
From pharmaceuticals to food processing, HCOOCH2H2O plays a significant role.
Yet, it’s crucial to approach this compound with caution. Proper handling ensures safety while reaping its benefits.
As research continues, we may uncover even more applications that could change the landscape of multiple fields.
Staying informed about new findings can provide insights into how best to utilize this chemical effectively and safely.
Conclusion
HCOOCH2H2O is a fascinating compound with unique properties and behaviors in aqueous solutions. Understanding its chemical characteristics, solubility, and impact on pH levels can open doors to various industrial applications. Its versatility makes it valuable across several sectors—be it pharmaceuticals, agriculture, or food production.
However, it’s equally important to prioritize safety when handling HCOOCH2H2O. Awareness of the necessary precautions ensures that we can harness its benefits while minimizing risks.
As research continues to evolve around this compound, its significance only seems poised to grow. Embracing the knowledge about HCOOCH2H2O will help professionals leverage its potential responsibly for future advancements.